Nov 01, 2024
Media
[Interview] JD Bioscience advances dual-action NASH drug as industry giants falte
본문
Before the U.S. FDA granted approval for Rezdiffra (resmetirom) as the first drug to achieve nonalcoholic steatohepatitis (NASH) resolution and fibrosis improvement in a phase 3 trial, the road to treatment was riddled with setbacks.
Intercept Pharmaceuticals’ Ocaliva (obeticholic acid), once a frontrunner, faced a second rejection from the FDA just last year. Major players like Pfizer and NGM Biopharmaceuticals also stumbled in their attempts to secure approval for therapies targeting this progressive liver disease.
“This situation provided us with an opportunity,” said Ahn Jin-hee, CEO of JD Bioscience, a Korean biotech startup founded in 2017 that focuses on metabolic diseases, in an interview with Korea Biomedical Review on Oct. 22.
Despite resmetirom's recent approval, Ahn remains undeterred, believing JD Bioscience is charting its own course with its NASH and fibrosis drug candidate, GM-60106, which completed phase 1 trials in April.
Resmetirom, a liver-directed, thyroid hormone receptor beta-selective agonist, has skillfully navigated regulatory hurdles. Yet Ahn points out a critical flaw: “While it effectively reduces liver fat,” he said, “its limited efficacy in improving fibrosis leaves an opening for new contenders.”

Ahn Jin-hee, CEO of JD Bioscience, emphasized the company's competitive advantage in treating nonalcoholic steatohepatitis (NASH) with their drug candidate GM-60106 in an online interview with Korea Biomedical Review (KBR) on Oct. 22. (Credit: KBR)
With GM-60106's “unique dual-mode mechanism of action and superior efficacy in addressing fibrosis,” Ahn is confident JD Bioscience has a competitive edge.
“When asked what is most important in drug development, I would say it is having a good target,” said Ahn, who noted that his company was founded in collaboration with physician-scientists focused on such targets, particularly in novel modalities.
GM-60106 prevents hepatic stellate cells in the liver from becoming activated, thereby blocking fibrosis while simultaneously inhibiting lipogenesis—the accumulation of fat in the liver. "This dual mechanism of action improves fibrosis outcomes," Ahn said.
A 2018 study published in Nature by Kim Ha-il, co-founder of JD Bioscience, identified a new potential therapeutic approach for NASH and liver fibrosis by targeting serotonin signaling between the gut and liver, leading to the development of GM-60106.
While resmetirom activates thyroid hormone receptors in the liver to reduce fat and inflammation, JD Bioscience’s approach centers on blocking serotonin production and signaling. Gut-derived serotonin can travel through the bloodstream to the liver, contributing to fat accumulation. This method particularly focuses on gut enzyme TPH1 and liver receptor 5-hydroxytryptamine receptor 2A (HTR2A,) which may reduce liver fat and potentially lessen inflammation and fibrosis without broader metabolic side effects.
In NASH, liver fibrosis is often driven by chronic inflammation and fat buildup. Since serotonin signaling seems to play a role in these processes, blocking serotonin receptors could potentially halt or even reverse fibrosis by targeting an upstream factor in the progression of NASH. Ahn highlighted this distinction, noting that "targeting a different pathway other than thyroid hormone receptors could offer complementary or alternative benefits, especially for patients who may not respond to resmetirom."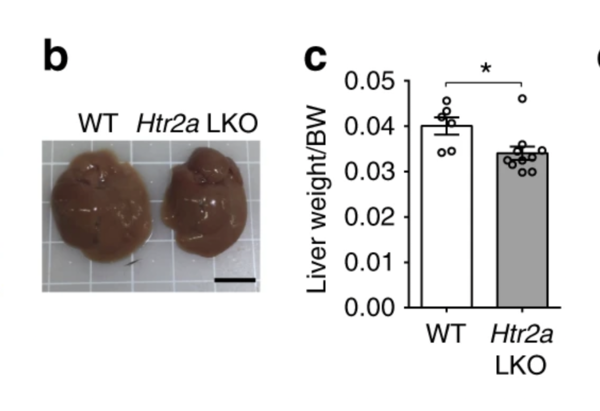
Livers of 12-week-old wild-type (WT) and HTR2A knockout (LKO) mice after 8 weeks on a standard or high-fat diet (b), along with the weight differences of their livers (c). (Source: Nature)
GM-60106 completed phase 1 clinical trials in Australia, with no serious drug-related side effects reported. JD Bioscience is now seeking approval for phase 2 clinical trials in the U.S. The company submitted its application on Sept. 23, with responses expected by Nov. 23. If approved, Ahn said phase 2 trials could begin in the first half of next year.
Ahn acknowledged the challenges of being a small biotech firm, noting that phase 1 trials cost nearly 10 billion won ($7.3 million) and phase 2 expenses are projected to exceed that amount. “Due to the high costs, we are actively seeking partnerships for technology transfer or co-development,” he added.
In its early stages, JD Bioscience secured a 5.1 billion won investment through the Accelerator Investment-Driven Tech Incubator Program for Startups (TIPS), allowing it to develop its candidate compounds. By 2019, the company had identified its first candidate and entered the preclinical phase, backed by Mirae Asset as a lead investor. Following successful preclinical studies, JD Bioscience completed a Series B funding round, raising about 20 billion won.
Ahn said the recent phase 1 trial in Australia was chosen for the country’s cost efficiency, as clinical trial approval processes in Korea are notably more complex and expensive. Even then, Ahn noted that the clinical trial costs were prohibitively expensive. “The initial estimate for the Australian trials was around 5 billion won, but final costs nearly doubled,” he said.
JD Bioscience has scheduled a meeting with its major shareholders on Thursday to discuss its Series C funding needs for ongoing clinical trials and research into its pipeline.
출처 : KBR(https://www.koreabiomed.com/news/articleView.html?idxno=25559)


